Acetic or Ethanoic Acid
Online Biology Dictionary
|
|
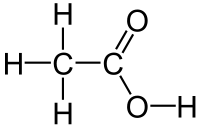 Molecular formula
Molecular formula
Acetic or ethanoic acid is the weak organic acid in vinegar. Acetic acid is the common name of this acid, while ethanoic is its systematic name (following IUPAC guidelines). In chemistry, a systematic name describes the chemical structure of a substance (which provides information about its chemical properties), whereas the common name is what the substance is called in everyday speech.
When pure and water-free (glacial acetic acid), it is colorless, hygroscopic, and freezes at 16.7°C (62°F). The acetyl group, a derivative of acetic acid, is basic in the biochemistry of nearly all organisms. When bonded to coenzyme A, it is essential to carbohydrate and fat metabolism. Acetic acid is produced by zymotic bacteria, in particular by microbes of the genera Acetobacter and Clostridium. Such bacteria are nearly ubiquitous and produce the sour smell and taste of spoiling food.
Most shared on Macroevolution.net:
Human Origins: Are we hybrids?
On the Origins of New Forms of Life
Mammalian Hybrids
Cat-rabbit Hybrids: Fact or fiction?
Famous Biologists
Dog-cow Hybrids
Georges Cuvier: A Biography
Prothero: A Rebuttal
Branches of Biology
Dog-fox Hybrids