
The Hybrid Hypothesis
7: The Gorilla and the Koolokamba
|
The gorilla is something of a paradox on the African scene. One thinks one knows him very well. For a hundred years or more he has been killed, captured and imprisoned in zoos. His bones have been mounted in natural history museums everywhere, and he has always exerted a strong fascination upon scientists and romantics alike. He is the stereotyped monster of horror films and adventure books, and an obvious (though not perhaps strictly scientific) link with our ancestral past. Yet the fact is we know very little about gorillas.
—Alan Moorehead
No Room in the Ark |
(Continued from previous section)
This section considers the gorilla, its likely origin, and the nature of its relationship to humans and panins. It represents an effort to expand the theory of human origins presented in previous sections. After all, most biologists think the panins and the gorilla are our closest living relatives. So the nature of the connection between humans and gorillas is directly pertinent to the subject under discussion. For many years, there was controversy over whether gorillas or panins (chimpanzees and bonobos) are more closely related to humans. The question was which pair—panins and humans, or gorillas and humans—shared the most recent common ancestor. The difficulty was that gorillas share certain traits with humans that panins do not and vice versa: panins have certain human characteristics gorillas don't. Recently scientific consensus has come down on the side of panins, and the general belief now is that panins are a little more closely related to the humans than is the gorilla—but only a little (and there are some primatologists who still disagree).
But from the perspective of stabilization theory, which, of course, holds that new forms of life typically come into being via stabilization processes associated with hybridization, the entire notion of divergence from a common ancestor makes no sense. That point of view suggests that the connection between Homo, Gorilla, and Pan would likely be of an entirely different nature, and that their origins would in some way involve hybridization. The previous sections have presented a strong case for the idea that humans are derived from hybridization between panins and pigs. The human case, then, lends credence to the idea that the gorilla, too, might be of hybrid origin, the question examined in the first half of this section. So does the fact of many other forms of life having had a hybrid origin, as documented elsewhere on this website.
With the exception of Pan paniscus, the gorilla was the last of the apes to be recognized, remaining unknown to European science until 1847, when its existence was first reported by Thomas Staughton Savage (1804-1880), who obtained several gorilla skulls in what is now coastal Gabon (Savage 1847a, 1847b). During the intervening years, much has been learned about this now famous animal, but in all ways, our knowledge of Gorilla gorilla is sparse in comparison with what we know of Homo sapiens, or even with what we know about chimpanzees and bonobos. The meager supply of human fossils seems abundant when one considers that paleontological remains of the gorilla are practically nil (Dixson 1981; McHenry and Corruccini 1980). Because of its rarity, both in the wild and in captivity, its intimidating size, and its inaccessible habitat, the gorilla has not been so readily available to researchers as have panins and most other primates.
Notwithstanding the absence of a fossil record, one can still consider the implications of reports made about the living animal in the years since its discovery. The gorilla's infertility and morphologically variable spermatozoa (see appendix) have not been satisfactorily explained by those who suggest that the presence of the such traits in a close relative (Homo sapiens) makes it "normal." Nor is any real elucidation provided by the claim that dysfunctional semen has "somehow" been favored by natural selection—this trait is an obvious impediment to reproduction. For my own part, I can only take the observations of infertility in humans and gorillas as evidence that their reproductive systems have somehow been disturbed. In the case of human beings, the evidence is consistent with the idea that the observed symptoms of infertility are symptomatic of hybridity. Why seek a different explanation for the gorilla?
So let's consider the possibility that the gorilla came into being via hybridization and attempt to identify the two types of animals that crossed to produce it. The procedure will be the same as it was in the case of human beings. Due to their close taxonomic affinity of panins to gorillas, it's reasonable to hypothesize that such an animal would have been one of the parents participating in such a cross.
The infertility of the gorilla is, if anything, more severe than that of human beings, which is consistent with the idea that the cross producing the gorilla was at least as distant as the one we have posited for human beings. This inference is seconded by another line of reasoning. The assumption that a panin crossed with some other type of animal to produce the gorilla entails the further assumption that the cross involved was a broad one: If this clause is excluded from the contract, the second parent has to be some type of primate. But then, what primate would it be? Because panins are so much smaller than gorillas, the second parent would almost certainly have to be very large, as large or larger than the gorilla itself. Otherwise the gorilla would not be intermediate in size between its parents, as is usually the case with a hybrid. The gorilla is far larger than any other primate. No primate can therefore be cast in the role of second parent for this cross unless we wish to assume that two little animals crossed to produce a giant.
Thus, if hybridity is to be an explanation of gorilla infertility, we are compelled to search outside Order Primates. Actually, we have already seen a great deal of evidence consistent with the idea that just such a cross produced the human race. And a similar approach in the case of the gorilla turns out to be informative as well.
If one grants, then, that it's reasonable to seek a nonprimate to play the role of the second parent, the next step is to compile a list of traits distinguishing the gorilla from panins. When one compiles such a list, and considers the geographic range of the gorilla, the most likely candidate turns out to be an animal that has done a better job of escaping the eye of science than any other large land mammal on the planet: Hylochoerus meinertzhageni, the giant forest hog (see photos and video below).
As will be shown, characteristics that distinguish gorillas from panins can be found consistently in Hylochoerus — at least to the extent that they can be verified in such a poorly researched animal. In short, then, the hypothesis under consideration becomes that not only humans are derived from hybridization between panins and pigs, but also that gorillas are derived from crossing of panins with a different kind of pig, resembling H. meinertzhageni.
Certainly, the forest pig is big enough to account for the huge size of the gorilla. Hylochoerus really is a giant. An average weight of 230 kg (507 lb) makes it the largest extant pig, which compares with 153 kg (337 lb) for an average male gorilla (Kingdon 1979, p. 211; Dixson 1981, p. 33).
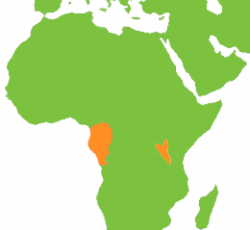
Even today, the gorilla is found only in geographic regions where the range of P. troglodytes and H. meinertzhageni overlap. Two gorilla populations exist in Africa, one on the west coast—bounded by the Cross, Congo, and Oubangui rivers—and a smaller population located about 600 miles away in eastern Democratic Republic of Congo (Wolfheim 1983). (See map at right.) P. troglodytes has a far larger range that stretches from Senegal to Tanzania, while P. paniscus is confined to a southern enclave defined by the Congo and Lualaba rivers (see map below). The two gorilla ranges lie entirely within the range of P. troglodytes so that wherever gorillas are found, both P. troglodytes and H. meinertzhageni are, as well. The gorilla and giant forest hog frequent the same habitats and have similar diets (Dixson 1981; Kingdon 1979).
|
|
|
The distribution panins in Africa |
In fact, in the gorilla's habitat, there really is no other large animal that might by any stretch of the imagination mate with a panin, most large mammals are confined to the open grasslands. Few such mammals are present in the dense thickets and rain forests frequented by the gorilla. Elephants, hippopotamuses, leopards, okapis, an occasional buffalo, and the giant forest hog are about the only animals on the list.
It would even seem that, viewed from certain angles, H. meinertzhageni resembles a gorilla: Dian Fossey, author of Gorillas in the Mist, actually mistook a giant forest hog for a gorilla at close range in full daylight (perhaps its head was turned or obscured by brush). She sat about a hundred feet away, using binoculars to observe a large, stationary animal she took to be a gorilla. Not having seen the animal move in quite some time, she decided to investigate. "I awoke my guide and asked him to remain where he was so that I could creep closer to the sunbathing animal," writes Fossey (1983, p. 8), "I'll never forget the chagrin felt upon realizing that the 'gorilla' I had been observing for over an hour was actually a giant forest hog (Hylochoerus meinertzhageni)." Fossey (ibid., p. 9) had "near daily encounters" with the giant forest hog while she was out observing mountain gorillas. Other gorilla observers frequently mention accidental encounters with Hylochoerus living in the same habitat (Merfield 1956, p. 177; Raven 1936, p. 333; Schaller 1963, p. 300). Traps set for the forest hog often capture and kill gorillas instead (Schaller 1963, p. 324).
So, in this animal, we have a creature that lives in the same places, and bears some resemblance to, the gorilla, even eating similar foods. Moreover, morphological features distinguishing gorillas from panins can be consistently accounted for in the anatomy of the forest pig (or at least that of pigs in general in those cases where specific information for Hylochoerus is unavailable).
As in the case of human beings, I have compiled a list of traits distinguishing gorillas and panins. I have included as many such features as I have been able to identify. The list is necessarily shorter than that for humans, as the amount of information available for gorillas is, in comparison, minuscule.
In considering this list of traits, it is important to keep one fact in mind: if gorillas and humans are both derived from hybridization involving pigs, they would have some pig traits in common, but in other respects we would expect them to differ. Homo and Gorilla, then, would be more similar to each other, with respect to certain porcine traits, than to panins. But not every porcine trait present in one would be present in the other. So even if the gorilla is a hybrid of a type akin to that posited for humans (pig x panin), and thus had certain traits in common with pigs, there is still no reason to expect it to have the same set of porcine traits as do humans.
In particular, it is not necessarily expected, if gorillas are pig-panin hybrids, that they should have large brains. Gorillas lack key traits elsewhere posited as having made brain expansion possible in human beings: They have poorly vascularized skin, except on the palms, soles, and knuckle pads (Ellis and Montagna 1962, p. 82). Even if gorilla skin were suited to heat dissipation, the gorilla brain would not be able to take advantage of it—gorillas have relatively few emissary veins) (the number of emissary foramina in a gorilla skull is comparable to the number in a panin's), which precludes the possibility of cooling the brain in the way that humans do. It seems this crucial pig trait is one they happened not to inherit.
Now let's consider a list of traits distinguishing gorillas (and Hylochoerus) from panins.
Traits Distinguishing Gorillas from Panins
Sub-orbital warts. In Hylochoerus, says Ewer (1958, p. 152), "the sub-orbital wart is enormously hypertrophied [i.e., extremely large]. The bone of the zygoma [i.e., cheek bone] is roughened and thickened and provides a firm anchorage for it" (see also Bouet and Neuville 1930, p. 227). These gigantic swellings are visible beneath the eyes of the pigs in the photos above. Unlike other primates, gorillas sometimes also develop huge, suborbital growths (Schultz 1950, p. 252, Plate 116; Schultz and Starck 1977). They arise on one or both cheeks. In gorillas, as in giant forest hogs, the bone affected is the zygomatic arch. Primatologists have been unable to say why these bizarre structures are seen in the occasional gorilla. A relationship to the forest hog, however, would explain the mystery.
Sagittal crest. Mature male gorillas have a bony sagittal crest running down the center of the upper rear portion of skull, a feature that is uncharacteristic of panins. Mature Hylochoerus boars, however, are distinguished from other types of pigs by the presence of a ridge of bone down the center of the supraoccipital (Ewer 1970, pp. 48, 52); see also (Ewer 1958, p. 345; Thomas 1904, Plate XV).
Brow ridges. Brow ridges are more pronounced in gorillas than in panins, particularly in mature males (see gorilla photo above). According to Ewer, the bone over the eyes "thickens" in old Hylochoerus males (Ewer 1970, p. 44a).
Jaw. The gorilla's jaw is massive in comparison with a panin's, a fact that would be fully accounted for by a connection with the giant forest pig (Ewer 1958, 1970).
Eyes. As can be seen in the photos above, giant forest pigs have darkly pigmented eyes like those of gorillas and panins, not the light colored eyes of pigs and many humans.
Rhinarium. The gorilla's nostrils are bordered by a thick pad of tissue not present in panins (Duckworth 1915, p. 159). The nose of the giant forest hog, too, bears a thick tissue pad (rhinarium) around the nostrils (Ewer 1958, p. 152; also see photos above). The nose of the forest hog is unsuited for rooting because the nasal septum is composed of fused bone, instead of being cartilaginous like that of the ordinary pig and humans (Ewer 1958, p. 152 and Plate 3b). The gorilla also lacks a cartilaginous nose (Schultz 1973, p. 52).
Liver. The gorilla's liver is unusual for a primate. Elftman and Atkinson (1950, p. 199) observe that
Specifically, "the liver of the gorilla differs from those of the other large anthropoids and of man in the more marked tendency to subdivision of right and left lobes, a character which assigns a comparatively lowly place to the gorilla in a comparison of the higher primates based on the anatomy of the liver" (Duckworth 1915, p. 209). Descriptions of the gorilla liver sound suspiciously like those given of this organ for the domestic pig.
Canines. Adult male gorillas have enormously developed canine teeth (Duckworth 1915, p. 248). The giant forest hog has huge canines, far bigger even than a gorilla's (Thenius 1981, Fig. 2).
Molars. Gorilla molars are larger than those of panins, are longer from front to back, bear more prominent cusps, and have deep grooves on their sides (Duckworth 1915, pp. 223, 248; Groves 1986). These characteristics are also seen in Hylochoerus (Kingdon 1979; Thenius 1981).
Femur. The section on human bipedality discussed several attributes linking the human thigh bone with that of pigs. These traits are all absent in the gorilla. One way in which a gorilla's femur is similar to a pig's, however, is in its overall robustness, that is, it is extremely thick in proportion to its length (Raven 1950, p. 152, Plate 60).
Sexual dimorphism. The average chimpanzee (P. troglodytes) female is nine-tenths as large as a male (Dixson 1981, p. 35; Schultz 1968, pp. 125, 126, Table 7-1). Gorilla females are only about half the size of males(ibid.). This distinction of the gorilla is readily accounted for by reference to the forest hog; Hylochoerus shows more sexual dimorphism in body size than any pig (Kingdon 1979, p. 211).
Diet. The major component of the diet in both the gorilla and the forest hog is herbaceous vegetation (Dixson 1981, p. 111; Langer 1988, p. 137). For example, both eat bamboo and vines (Kingdon 1979, p. 214; Reynolds 1967, p. 146; Wolfheim 1983, p. 687). In the case of Hylochoerus, even grass can be consumed in quantity (Kingdon 1979). The foods of panins are more varied than those of the gorilla. Dixson (1981, p. 115) points out that "whereas gorillas favour secondary forests and feed mostly at, or near, ground level on leaves, pith, shoots, roots, etc., chimpanzees prefer primary forests where they climb a great deal and feed principally upon fruits." Panins supplement their diet with a variety of foods including leaves, insects, small animals, and eggs.
Social structure. Unlike panins, gorillas travel in large groups led by a big silverback male (Dixson 1981). Walker (1983, p. 1179) notes that the giant forest hog also goes about in sounders composed "of up to 20 individuals," and that they "are led by an old male." (See photo of a forest pig sounder above.)
Pigmentation. The gorilla is darkly pigmented throughout life. Even the youngest specimens are as dark as adults (Duckworth 1915). Panin skin is typically much lighter than that of the gorilla, particularly in juvenile specimens. The skin of Hylochoerus is also dark (Dorst and Dandelot 1970).
Hybrid Traits
Under the hypothesis that the gorilla is a hybrid, it is possible to attribute some traits to the mere fact of its hybridity:
• Abnormal spermatozoa
• Testicular atrophy
• Low sperm density, 4.1 × 107/ml (Gould and Kling 1982, p. 313), about one-sixtieth that (2.56 × 109/ml) seen in chimpanzees (Gould 1993, Table II).
• Low mtDNA variability in conjunction with high nuclear DNA variability.
• High levels of morphological and biochemical variability.
Porcine Characters Shared by Humans and Gorillas
Nasal bones. The gorilla has longer nasal bones than the other apes, as do human beings (Duckworth 1915, pp. 170-171; Keith 1899). As a result, the upper portion of its nose tends to form a somewhat elevated bridge (Duckworth 1915, Fig. 147; Keith 1899). Long nasal bones are also characteristic of Hylochoerus (Ewer 1958; 1970).
Short upper lip. A gorilla's upper lip, that is, the distance between the mouth and nostrils, is short in comparison with that of a panin (Duckworth 1915, p. 159). As in other pigs, the upper lip is short in Hylochoerus (Ewer 1970, Plates 1 and 2).
Hands and feet. In the shape of their hands and feet, gorillas, and not panins, are most similar to human beings:
• As in humans, the gorilla palm is wide in proportion to its length(Schultz 1968, pp. 134, 135, Fig. 7-6). "Man and gorilla are distinguished by the shortest hands of all higher primates" (Schultz 1936, p. 435). They also differ from the chimpanzee in having shorter hands than feet (Schultz, p. 127,Table 7-2, 1950, p. 233, Table 3; 1968, p. 127, Table 2).
• Although human toes and fingers are proportionately the shortest, the gorilla comes in a close second (Schultz 1950, 1968, pp. 131-132); See also (Raven 1950, pp. 154-155, Plates 62 & 63).
• Although still opposable, the big toe is, as in humans, more extensively connected with the main body of the foot (Dixson 1981; Schultz 1950). The other toes also tend to be webbed together to a much greater degree (and are much shorter) than in panins. The thumb and great toe are about the same size in proportion to body size (trunk length) in panins, in gorillas, and in human beings (Schultz 1936, Figs. 16, 17; 1968, p. 127, Table 2 & pp. 135, 163). It is only the other digits that are markedly shorter in gorillas and human beings (Schultz 1936, pp. 435a, 439b).
• While human beings have the longest heel bone in proportion to foot length of any primate, the gorilla has the second longest.
Brachial index. Forearm length as a percentage of upper arm length is about 80 percent in both humans and gorillas, the smallest value among catarrhine primates. The humerus is also longer than the radius in pigs.
Terrestrialism. Gorillas, particularly adult gorillas, are almost exclusively terrestrial, a trait shared with human beings and pigs, but with few other primates.
Malleolus medialis. This downward directed spike, present on the lower end of the tibia in other nonhuman primates, is absent in the gorilla (Raven 1950, p. 153, Plate 61), as it is in humans and pigs.
Melanocytes. Ellis and Montagna (1962, p. 81) note that "In spite of the heavy pigmentation of the [gorilla] epidermis, there are no dermal melanocytes." This trait links gorillas with humans and pigs to the exclusion of panins and monkeys (see the previous page). As Montagna (1972, p. 115) notes, "Dermal pigment occurs normally in most primates." So its absence from the dermis in the heavily pigmented skin of the gorilla is unusual.
Hymen. Raven (1950, p. 89b) notes the presence of a well-defined hymen in the gorilla (see also Gerhardt 1906). This structure is not found in panins nor, apparently, in other nonhuman primates (Sonntag 1924, p. 269). I have not found descriptions of the genitals of the forest hog, but in the ordinary pig (and various other ungulates) a hymen is present in virgins (Nickel et al. 1973, pp. 376-377).
Labia. "In the gorilla the labia majora are distinct but small, the labia minora absent" (Raven 1950, p. 89b) . This configuration is the opposite of that found in other nonhuman primates, but the same as that found in domestic pigs (see the previous page).
Sexual swellings. Neither human beings nor gorillas have the conspicuous sexual swellings seen in the females of most other nonhuman primates, including panins (Merfield 1956, p. 53; Schultz 1968, p. 142). These swellings are not characteristic of pigs.
Menstrual cycle. Chimpanzees have a cycle length of 36-37 days; the human cycle is about 28 days long; that of pigs, 21 days. A gorilla's, which is 31-32 days long (Nadler 1975, 1976), is also shorter than a panin's.
The gorilla, then, shares quite a few non-panin traits with humans and pigs. This commonality is explained by the hypothesis that both humans and gorillas are derived from hybridization between panins and pigs, albeit to different kinds of pigs. Other traits, however, link gorillas, not with humans, but with panins and other nonhuman primates. Characteristics in this second category have all been mentioned in previous sections, but they can be summarized briefly here:
Nonhuman Traits Seen in both Gorillas and Panins:
Gorillas and panins are linked by: a thin epidermis, a high intermembral index, a long pelvis with a large birth canal, a short coccyx, poorly vascularized skin containing few elastic fibers, small brains, dark eye pigmentation, non-circular prostate, fear of water, brief copulation, and identical chromosome counts.
Both gorillas and panins lack numerous human features which have already been covered in previous sections: a panniculus adiposus, a gap between pelvis and rib cage, a lumbo-sacral promontory, a centralized spine, a projecting cartilaginous nose, a philtrum, a chin, a diverticulum in the stomach, a styloid process, knock knees, epidermal patterning (external and internal), heavy gluteal muscles, seventh cervical vertebra with prominent spine and transverse foramens, ear lobes, thermoregulatory sweating, bulbo-urethral glands, ungual tuberosities, glabrous lips, valvulae conniventes, multipyramidal kidneys, bunodont molars, nocturnal activity, and female orgasm, as well as susceptibility to flea infestation, sunburn, alcoholism, melanoma, atherosclerosis, and heart attack.
Both have traits that humans and Sus lack: a panniculus carnosus, a hairy pelt, knuckle pads, brow ridges, non-divergent eyes, long dorsal spines on cervical and sacral vertebrae, various features of the femur distinguishing chimpanzees from humans (circular condyles of unequal size, shallow intercondylar notch, shallow patellar groove with high medial lip), circular lateral menisci in the knees, laryngeal sacs, and a baculum.
Hybridization between Gorillas and Chimpanzees
Continuing hybridization of gorillas and panins would have an influence on the panin population itself even though it is far larger than the gorilla population (a large population tends to be less affected by hybridization than a small one). This effect would, presumably, be limited to panins north of the Congo-Lualaba river barrier, as the gorilla and the giant forest hog occur only north of this barrier. Therefore the phenotype characteristic of the common chimpanzee (P. troglodytes) may be the result of such hybridization, whereas that characteristic of bonobos (P. paniscus) may approach, or even be identical to, the phenotype that typified panins prior to their first hybridization with pigs (since the bonobo occurs only to the south of the river barrier and would therfore be shielded from any genetic interaction with the gorilla or forest hog).
Pan paniscus, is, in fact, much less variable than P. troglodytes, which also fits with the idea that the former has been protected from hybridization (populations affected by hybridization are generally more variable than those that are not). It is generally treated as a monotypic species; there are no recognized subgroups or races (Wolfheim 1983, p. 701). This lack of variability in comparison with P. troglodytes, which is commonly asserted to have three, or even four, different subspecies, is not merely with respect to external features. It extends also to a variety of less obvious characteristics, ranging from sperm morphology (Seuánez et al. 1977), to skeletal measurements, to cranial features (Bragga 1993, Cramer 1977, Table V), to blood groups and other genetic markers (Goodman et al. 1970; Lucotte 1981; Moor-Jankowski et al. 1972, 1975, p. 266; Socha 1984, pp. 35-39; Weiner et al. 1973).
But, beyond the fact that P. troglodytes is more variable, there is the interesting fact that these northern panins tend to be more like gorillas. Beyond the fact that bonobos are smaller than common chimpanzees—85 percent as big (Corruccini and McHenry 1979; Zihlman and Cramer 1978, p. 362)—various other traits characteristic only of P. troglodytes, such as large canines and robust jaws, are consistent with gorilla ancestry. Traits that are never seen in bonobos (Fenart and Deblock 1973)—for example gorilla-like noses, sagittal crests—are in fact reported, albeit rarely, for panins living in regions where the gorilla is also present (Angst 1967; Cramer 1977, p. 43; Frechkop and Marit 1968; Groves 1970, p. 12; Merfield 1956, p. 52; Schultz 1969, p. 56). Most telling, however, is the fact that numerous researchers have reported wild-caught apes of indeterminate status, having traits intermediate between those ordinarilly characteristic of Gorilla gorilla and P. troglodytes. These reports suggest the existence of ongoing hybridization between gorillas and chimpanzees even at the present day. Reports relevant to these intermediate apes are so numerous and have persisted for so many years that it would be surprising if a genetic survey of the pertinent geographic regions failed to confirm what available reports already strongly suggest. In the remaining pages of this section these reports have been condensed into a history—that of an intermediate ape, the koolokamba.
The Koolokamba: A Probable Natural Hybrid of Gorilla and Chimpanzee
For more than a century, naturalists have been aware that the indigenous peoples of equatorial Africa claim that hybridization occurs between gorillas and chimpanzees. And yet, the generally accepted view of science seems to be that such events do not and cannot occur, though A. F. Dixson (1981, p. 19), a leading authority on the gorilla, did assert that "a cross between the two might be feasible in captivity, if artificial insemination were used." On its face, any assertion that they simply cannot cross is somewhat strange, since there is no known anatomical or physiological impediment. They have the same chromosome counts (2n = 48), and indeed, in the author’s experience, many other organisms differing far more at the genetic level can in fact produce offspring together.
But, beyond such speculation, a great deal of hard evidence exists to support the idea that natural hybridization does occur, far more evidence, in fact, than there is for many other mammalian crosses that are well accepted, which is puzzling. Has the near relation of these apes to human beings perhaps led biologists to overlook facts that would be completely convincing in the case of other kinds of crosses?
In particular, there have been many reports about chimpanzees with gorilla-like traits. These are individuals with traits intermediate in between those of chimpanzees and gorillas. In some cases the intermediacy of these specimens has been such that expert primatologists have had trouble classifying them definitely as either chimpanzees or gorillas. Some naturalists have interpreted these chimpanzee-gorilla intermediates as hybrids, while others have claimed they are merely chimpanzee "variants" (that is, ordinary chimpanzees that are gorilla-like, but that nevertheless fall within the ordinary range of variation for chimpanzees).
Those who study natural hybridization know that it is common for disputes of this sort to arise when specimens of an intermediate nature ("intermediates" as they are often called) are found in a natural setting. It is in fact typical in such cases, for some experts to say the specimens are hybrids, and for others to say they are variants. However, the standard method of resolving such debates is to determine where the intermediate specimens are coming from. Locality is key: If the question is examined geographically and the specimens have all been found in areas where the two putative parents come into potential breeding contact, then it makes sense to conclude that the specimens are hybrids. For if the unusual individuals were variants of a given type of organism A, and not hybrids arising from matings between organism A and organism B, then they would be expected to occur at random throughout the range of A, and not just in the part of A’s range where A comes into contact with B. This reasoning is straightforward.
But to make a discrimination of this kind, it is necessary to look at the origins of the intermediate specimens, preferably all the intermediate specimens — which can be a lot of work. But if they do all come from regions where A comes into contact with B, it becomes obvious that they are hybrids. On the other hand, if it is found that they occur at random within the range of A, it's reasonable to suppose they are variants.
We will apply this same method to the case currently under consideration, that of the chimpanzee and the gorilla. Doing so requires an examination of all available reports of such animals ("gorilla-like chimpanzees," as they are usually described), in order to determine the geographic locales where each was found. A detailed examination of this question follows below, but suffice it to say here that the finding of a detailed investigation of this matter indicates that all such intermediate animals have, in fact, come from areas where both gorillas and chimpanzees occur. This fact alone, in the case of any other cross, would constitute conclusive evidence that hybridization does occur. Therefore, it does also in the case of gorillas and chimpanzees. So we may safely conclude that gorilla-chimpanzee hybrids do occur in the wilds of equatorial Africa. But for those desiring details may wish to read on. The following narrative provides the relevant facts, documented in the form of a history of what has been reported about chimpanzee-gorilla intermediates, that is, about chimpanzee-gorilla hybrids.
Reports of Chimpanzee-Gorilla Intermediates: Only five years after the discovery of the gorilla itself, Franquet (1852) reported that the indigenous peoples of the Gabon (the region of western equatorial Africa adjacent to the Gabon River) believed that gorillas cross with chimpanzees to produce a hybrid ape of intermediate form. A few years later, explorer Paul Du Chaillu (1861a) shot an ape and sent its skull to the British Museum from the Gabon—a region where both chimpanzees and gorillas were present. This male specimen was intermediate in character between the skulls of ordinary gorillas and chimpanzees. It was "smaller than the adult male gorilla and stouter than the female gorilla" (Du Chaillu 1861b, p. 408). Du Chaillu suggested that it should be treated as a separate species of chimpanzee, to which he gave the scientific name Troglodytes kooloo-kamba, an epithet derived from one of the local dialects. Koolokamba is the name used since in most papers dealing with this creature (a variety of spellings appear in the literature (kooloo-kamba, kulu kampa, kouloukamba, etc., but the most common is koolokamba).
The names given this animal in the languages of equatorial Africa, confirm that the locals think it is a hybrid. As Cousins (1980, p. 91) notes, "The local names given by the indigenes vary, but all appear to agree on the basic meaning of the description: chimpanzee-gorilla or gorilla-chimpanzee." These aliases occasionally sneak into the scientific literature. Koula-nguia is the term used by Raingeard (1938), but he obviously refers to the same animal: "In addition to the gorilla and chimpanzee, the indigenous peoples of the Gabon distinguish a third ape with habits very different from those of the other two. Chimpanzee-gorilla is the name given it in the various dialects" (Raingeard 1938, p. 81) — Koula-nguia, means "chimpanzee-gorilla" in the Akele language (ibid). The Fang people of equatorial Guinea use the term N’gui-Moun which means gorilla-chimpanzee (Cousins 1980, p. 88). Strictly speaking, however, this ape’s name is not derived in every dialect from the words for chimpanzee and gorilla. Good (1947, p. 45) writes that
|
|
| Mafuka |
According to Keith (1899, p. 296), the earliest such intermediate to be dissected was a specimen described under the name Troglodytes aubryi by Gratiolet and Alix (1866). During the ensuing half century, several of these peculiar apes made their appearance in Europe. The best known was a female ape named "Mafuka," residing in the Dresden Zoo (see figure right). In 1877, primatologist A. B. Meyer (1877) asserted that Mafuka was a hybrid of chimpanzee and gorilla. Meyer’s paper "initiated a storm of controversy" that ape expert W. C. O. Hill (1969, pp. 23-24) claimed, nearly a century later, had "not yet been finally settled."
Six years after Meyer’s assertion, another expert on anthropoid apes, Robert Hartmann (1886, pp. 215-219), discussed the Mafuka dispute: "When I first saw this savage creature, early in September, 1875, it was full of vigour, and I was almost convinced that I saw a female gorilla, not quite an adult, an opinion shared by such zoologists as K. Th. von Siebold and others, while it is vehemently opposed by Bolau [1876] and A. B. Meyer [1877]." Hartmann goes on (p. 220) to express his own doubts about Mafuka’s classification. In the end, he wrote: "For me and many other naturalists Mafuka remains up to this time an enigma, which is slurred over by others with a few phrases." At one point (pp. 218-219), he admits having leaned toward the idea that she was a hybrid:
Before going on with this story, it’s worth mentioning that J. C. G. Allen, in his Gorilla hunting in southern Nigeria, also reported mixed groups of wild chimpanzees and gorillas feeding and playing together. Gorilla expert A. F. Dixson (1981, p. 19) also says, "that kooloo-kambas have been observed in normal groups of chimpanzees, which suggests a cross between a male gorilla, perhaps a lone silverback, and a female chimpanzee." According to Meyer (1881), this was the direction pictured for the cross by von Koppenfels, who thought the careful eye that male gorillas keep over their harems is too vigilant for a male chimpanzee too slip in and carry out the required insemination. Meyer himself, however, felt that a cross between an old chimpanzee male and young gorilla female would be more plausible because the size difference would be minimized. And it can be pointed out, too, that the time from intromission to ejaculation is very short for a male chimpanzee—on the order of ten seconds.
Be that as it may, nearly a century later Groves (1970a, p. 19) continued the story of von Koppenfels’ "hybrids" (or of Meyer’s "chimpanzees"):
Elliot (1913, p. 224) compared his proposed "Pseudogorilla" with the other African apes as follows:
But Groves (1970a, p. 19) dismissed the idea that these animals were hybrids or that they perhaps deserved treatment as a distinct species:
Grove’s use of the word young here is hard to understand, since Elliot (1913, p. 225) says the middle of this male’s back, its flanks and the hinder parts of its thighs were iron gray. Grey hair on the back is the mark of maturity in male gorillas. Gorilla troops are usually led by a mature "silverback," as such individuals are called. Dixson (1981, p. 31), a leading expert on gorillas, says that as silverbacks age "the grey areas spread to include the flanks and buttocks," the same regions where Elliot’s specimen was gray.
The specimens "discovered" by Elliot in 1913 are apparently the very same "cross-breeds" sent to A. B. Meyer some thirty years earlier by von Koppenfels. Elliot’s specimens had been shot on the Fernan Vaz, an estuary on the Gabon coast (where both chimpanzees and gorillas exist) while in association with a troop of gorillas, and they were stored in a German museum. Von Koppenfels had 1) operated out of "Eliva Comi on the Fernan Vaz" (Meyer 1881, p. 231); 2) had shot his specimens in a troop of gorillas; and 3) sent them to Germany. If Elliot’s specimens were in fact the ones sent to Meyer by von Koppenfels, four different judges assigned them four different identities: 1) hybrids (von Koppenfels); 2) ordinary chimpanzees (Meyer); 3) "pseudogorillas" (Elliot); 4) ordinary gorillas (Groves).
Other intermediate apes continued to meander into scientific journals over the years. In 1898 we find W. L. H. Duckworth (1898, p. 989) at Cambridge University puzzling over a preserved carcass he had just received from Africa:
In his article he compares his specimen to several other apes (some of these also of indeterminate status), and points out that his ape has many chimpanzee features. But, ultimately, although he rejects an identification of the specimen as an ordinary gorilla, he also says (Duckworth 1898, p. 994),
One of the other intermediate apes mentioned by Duckworth (1898, p. 994), "Johanna" of the Barnum and Bailey Circus, is discussed at length by primate anatomist Arthur Keith. While Johanna’s owners claimed she was a gorilla, Keith (1899, p. 296) claimed that she was a chimpanzee — or rather, a koolokamba:
In the 1920’s Charles Sonntag was one of the world’s foremost authorities on chimpanzee morphology (Sonntag 1923). In 1924, he brought together in a single volume what was then known about primate anatomy (The Morphology and Evolution of the Apes and Man). In it he lists many features by which the African apes can be distinguished from each other, but he includes a caveat (p. 86): "It occasionally happens, however, that it is difficult or even impossible to tell whether a particular animal is a gorilla or a chimpanzee…[and] when a difficulty arises, it is in connection with the females." This assertion was echoed 56 years later by Cousins (1980, p. 89): "It is a strange coincidence that so far all captive chimpanzees described as Koolookambas have been females." It does seem that females predominate in the literature, though a few males have been reported. However, this predominance is not at all surprising if these animals are interpreted as hybrids, in fact it constitutes a strong corroboration of the hypothesis: According to Haldane’s Rule, when in the offspring of a cross between two mammals one sex is absent or rare, it is expected to be the male. It is only if we interpret them as ordinary chimpanzees that it becomes difficult to see why nearly all of the many reported intermediate specimens should be female. Under that hypothesis we would expect the male-female ratio to be near 50-50.
Nevertheless, Ernst Schwarz (1934a) not only denied that koolokambas are hybrids, but even refused to admit that they exist as entities distinct from the chimpanzee. He argued against the idea that specimens described as koolokambas should prompt the erection of a separate species (or subspecies), and insisted they were mere variants of the common chimpanzee. When two more possible chimpanzee-gorilla hybrids surfaced in the literature four years later (Raingeard 1938), Schwarz (1939) didn’t change his opinion. He promptly pounced on them, too, saying he had "no doubt that the ‘koula-nguia’ was only a black-faced chimpanzee."
In saying that Raingeard’s specimens were "only" black-faced chimpanzees, Schwarz implies they were ordinary chimpanzees, but this conclusion does not follow: In Cameroon, many apes passed through the hands of veteran collector Fred Merfield (1956, pp. 52-53) on their way to zoos overseas. One of them was named Bo-Bo.
Although Schwarz was a brilliant zoologist, he might be better described as a mammalogist than a primatologist. Primatology, however, was certainly the calling of W. C. O. Hill. Schwarz wrote a blizzard of articles on a wide variety of mammals; Hill’s major work was the monumental, multi-volume Primates: Comparative Anatomy and Taxonomy. So if anyone could judge what constituted the unusual in a chimpanzee, it was Hill. He did not consider the matter of the koolokamba so cut-and-dried as Schwarz, and he clearly expressed his opinion (Hill 1969, p. 35) that "review of the literature and personal experiences" had convinced him "of the general validity of Schwarz’s results [in revamping the taxonomy of the chimpanzee], but a lingering doubt remained, amounting almost to a tacit belief, in the existence of two races inhabiting the Lower Guinea region" (see map). In a separate paper (Hill 1967), he expressed his view in more detail:
Rode’s suspicions that the koolokamba might be a distinct race of chimpanzee were based in part on a study of two unusual skulls, one from Congo, the other from Gabon. Quoted in Malbrant and MacLatchy (1949, p. 58) concurred with this view and quoted Rode as follows:
Similar taxonomic woes were voiced by famous ape experts Ada and Robert Yerkes (Yerkes and Yerkes 1945, p. 393), who also noted that it can sometimes be difficult to tell gorillas from chimpanzees:
Louis de Lassaletta (1958) describes yet another intermediate ape, shot in the Nsork Rain Forest in Rio Muni (modern Equatorial Guinea) in 1958. More recently, primatologist R. V. Short (1980, p. 5) discussed the question of whether the koolokamba should be treated as a subspecies of the chimpanzee, and concluded that
Short seemed particularly impressed with the koolokamba skull sent to the British Museum by Du Chaillu. Merfield had also pondered the question of whether koolokambas (or "chogas" as he called them) constitute a separate race. Although he was at one time convinced that they do, he seems ultimately to have recanted. After finding one of these animals in a troop of ordinary chimpanzees, he became an apostate, stating in his field notes (quoted in Shea 1984, p. 8) that "it shakes my faith as to really black-faced chimps or chogas being a separate race."
But is there really any reason to speak of a separate "race" of chimpanzees? The very rarity of these specimens is consistent with the idea that koolokambas are either hybrids or unusual variants. Of 141 chimpanzees in a collection examined by Reynolds and Luscombe, only two were koolokambas, and this appears to be a larger representation than is typical of most chimpanzee colonies (Reynolds and Luscombe 1971, Table 1). These authors (ibid., p. 131) cautioned against Hill’s tentative treatment of P. t. koolokamba as a subspecies:
The crucial point is that these "variants" are not reported in chimpanzee populations where the opportunity to hybridize with gorillas is lacking. All of these intermediate apes — at least those of known origin — come from regions where not only chimpanzees, but also gorillas, are present (see Table 8.1). Throughout the length of the west coast of equatorial Africa, from Senegal to the Congo, chimpanzee collection has been intense for many years (Wolfheim 1983, pp. 709-710). And yet, the vast majority of these intermediate apes have been reported only in the region Hill (1969, p. 41) calls "Lower Guinea," which is the portion of the west coast where gorillas are present (see map above). None have been reported from other west coast countries where there are no gorillas, such as Sierra Leone, where chimpanzees have been heavily exported for more than a century. For example, in one five-year period (January 1973 - April 1978), 1395 chimpanzees were shipped from Sierra Leone (Short 1980, p. 6). Among so many specimens, why no "variants"?
Chimpanzee-gorilla intermediates are still being seen today. In recent email correspondence with wildlife photographer and anti-bushmeat activist Karl Ammann, I have received photos of two such apes, now in his possession, but originally from the northern forests of Democratic Republic of Congo. He also has pictures of a crested "chimpanzee" skull on his website. Ammann also says that he knows of at least four such apes currently in captivity in Africa.
The only reports of intermediate apes outside Lower Guinea are from areas in or adjacent to the eastern range of the gorilla in inland central Africa (Frechkop and Marit 1968; see also Hartmann 1886, pp. 223, 240, and Karl Ammann's intermediate apes, pictured in the box at right). But in comparison with coastal Lower Guinea, relatively few of these "chimpanzee variants" have been reported from this region—probably because, as Wolfheim (1983, p. 710) points out, few chimpanzees of any kind have been collected for export from the inaccessible reaches of the northeastern part of the Democratic Republic of Congo. Hartmann (1886, pp. 223, 240) does, however, mention a possible case from the eastern range, an ape found by Livingston in what is now the Maniéma district of modern Democratic Republic of Congo west of Lake Tanganyika. In the 1960s, Marit did capture two such specimens, "chimpanzees" with gorilla-like sagittal crests, in the eastern range of the gorilla near Kampene, in the Maniéma district (Frechkop and Marit 1968, p. 32). He and Frechkop then searched the extensive collection of chimpanzee skulls in the Musée Royale d’Afrique Centrale at Tervuren, Belgium. They found the skulls of ten more "chimpanzees" with heavy jaws and sagittal crests. Nine were of known origin — all from gorilla country, one from the western range, and eight from the eastern. The eastern range preponderates because the Tervuren specimens are mostly from what is now the Dem. Rep of Congo, which was formerly a Belgian colony, a region that does not include any part of the eastern range of the gorilla. The western skull was obtained in 1928 at Bomongo, just over the Oubangui River from the eastern limit (Cousins 1978) of the lowland gorilla’s range (Impfondo) and may have been carried over the river to Bomongo by humans. The six crested chimpanzee skulls mentioned by Cramer (1997, p. 43) are probably among the nine listed by Frechkop and Marit since Cramer (1997, p. 8) selected his specimens from the same collection.
If the numerous intermediate apes that have been reported are only chimpanzee variants and not hybrids of gorilla and chimpanzee, why are there no accounts of them from other parts of the chimpanzee’s extensive range where gorillas are absent? Why is it that "one finds chimpanzees with gorilla-like noses," as Groves (1970a, p. 21) observes, "quite commonly in Gabon and Cameroun [i.e., Lower Guinea]" and not elsewhere? Indeed, why are these "chimpanzee" variants consistently described as "gorilla-like" if gorillas are not in some way involved? The vast majority of genetic variants have only a single gene mutated. This is the typical case with albinos, the example given by Reynolds and Luscombe. It is unlikely that mutation of a single gene would alter enough characteristics to make a chimpanzee "gorilla-like."
Shea (1984, p. 10) suggests that these intermediate individuals simply reflect "an important biological reality, i.e., gorillas and chimpanzees are very closely related animals with patterns of morphological development which coincide and overlap." But again, this explanation does not account for the fact that these so-called variants are found only in regions where chimpanzees have the opportunity to hybridize with gorillas, which only represents about a third of the entire chimpanzee range. Although he attempts to account for koolokambas in terms of individual variation within, and overlap between gorilla and chimpanzee populations with respect to their physical traits, Shea himself points out (ibid) that the "congruence [between chimp and gorilla] is so great that the production of viable hybrids remains a real possibility." Indeed, as documented elsewhere on this website, P. paniscus and P. troglodytes have crossed in captivity.
In the case of animals less closely related to humans, hybridization is the first explanation that springs to the naturalist’s mind when intermediate specimens are observed. Nothing is more common than for a biologist to diagnose specimens as hybrid when they (1) have intermediate traits between two putative parents and (2) the specimens were obtained in a geographic region where the two proposed parents come into contact. Such diagnoses commonly occur where even a single trait (such as coat color or body size) indicates hybridity. For example, in considering specimens from a region where the Cotton Deer Mouse (Peromyscus gossypinus) and the White-footed Deer Mouse (P. leucopus) come into contact, Howell (1921, p. 50) diagnosed them as hybrids between the two on the sole basis of the fact that they had "decidedly larger skulls." In other respects, the specimens were similar only to P. leucopus, but in the present case, that of putative hybrids between the chimpanzee and gorilla, it is not merely the two facts that 1) the specimens come only from regions where both chimpanzees and gorillas are present and (2) the skulls are larger than those of ordinary chimpanzees. It is also the case that a variety of other traits are similar to those of gorillas in these supposed chimpanzees.
The evidence suggests that these intermediate apes actually are hybrids; if they are, the whole thrust of the debate has gone astray. Under such circumstances it would not be germane to debate whether a rare subspecies of chimpanzee exists in Lower Guinea and whether this unusual type deserves a special name. More than a hundred years ago, von Koppenfels wrote from Africa that he had "positive proof" that the koolokamba was a hybrid and "not a distinct species," and went on to say (von Koppenfels 1881, p. 448) that this "mongrel progeny of the male gorilla and female chimpanzee discovered by me, is found, but in individual cases, and as such, deserves no special name." If natural hybridization does in fact occur between gorillas and chimpanzees, von Koppenfel’s attitude is the only sensible one to take. Any further discussion of species and subspecies would be pointless.
And there is no reason to think that nucleotide sequence data will be particularly helpful in resolving this question. There is good reason to suppose, as there is in humans (see next section), that gorillas are backcross hybrids
Conclusion
We have seen evidence that the gorilla is of probable hybrid origin. A specific cross was proposed as a likely object of further investigation (chimpanzee × giant forest hog). The existence of apes intermediate between the gorilla and chimpanzee suggests that the northern chimpanzee population (P. troglodytes) continues to hybridize with the gorilla even today. In the typical hybrid zone, at least those where the hybrids are partially fertile, the influence of hybridization spreads outward into adjacent populations until a barrier of some type is reached. In the present case, the barrier would be the Congo and Lualaba rivers. Beyond this barrier, insulated from the genetic influence of hybridization, is Pan paniscus, which is therefore expected to be closer to the primitive panin type. Hence, what we call Pan troglodytes seems itself to be a hybrid population of sorts: a population composed of individuals quite similar to P. paniscus, but somewhat influenced by interbreeding with the gorilla. And the traits in question seem to derive, ultimately, from H. meinertzhageni, since it appears likely that the gorilla is a derivative of crossing between P. troglodytes and H. meinertzhageni. So the picture that emerges is that humans, the common chimpanzee, and the gorilla all appear to be derivatives of hybridization. Under this view, P. paniscus is best extant representative of the "true" and original panin that predated the advent of H. sapiens, G. gorilla and P. troglodytes. Also, if the conclusions reached thus far are correct, the giant forest hog and the ordinary pig must also have existed before these three offspring forms into being. Therefore, we should use only P. paniscus as a standard of comparison in identifying human traits of porcine origin (at least when the trait in question differs between paniscus and troglodytes). This is a useful point to keep in mind as we fine-tune our analysis of human origins.
TABLE 8.1 Reported Intermediate Apes of Known OriginExamples from western range of the gorilla: |
||
1855 |
Duvernoy's Troglodytes tschégo |
Gabon |
1861 |
Du Chaillu's koolokamba |
Gabon |
1866 |
Gratiolet and Alix (T. aubryi) |
Gabon |
1877 |
Alix and Bouvier (Gorilla mayêma) |
Gabon |
1877 |
Mafuka |
Gabon |
1880s |
von Koppenfels' "cross-bred" animals |
Gabon |
1896 |
Garner's koolokamba |
Gabon? |
1898 |
Jena Museum ape (Duckworth) |
Gabon |
1898 |
Duckworth's specimen |
Gabon |
1900 |
Johanna (Keith) |
Gabon |
1913 |
Elliot's pseudogorilla |
Gabon |
1930s |
Merfield's chogas |
S.W. Cameroon |
1939 |
Raingeard's koula-nguias |
Gabon |
1948 |
Rode's koolokamba skulls |
Congo and Gabon |
1954 |
de Lassaletta's koolokamba |
Nsork rain forest, Equatorial Guinea |
1960 |
Denis's koolokamba |
Congo |
1970 |
Grove's gorilla-nosed chimps |
Gabon and Cameroon |
1980 |
Cousin's "Minnie" |
Cameroon |
Examples from eastern range of the gorilla: |
||
1870? |
Livingston's Soko |
Maniéma District, D.R. Congo |
1968 |
Frechkop and Marit (crested "chimps") |
D.R. Congo, Burundi |
2012 |
Karl Amman's Mzee and Bili (and crested "chimp" skulls shown on his website) |
northern D.R. Congo |
Most shared on Macroevolution.net:
Human Origins: Are we hybrids?
On the Origins of New Forms of Life
Mammalian Hybrids
Cat-rabbit Hybrids: Fact or fiction?
Famous Biologists
Dog-cow Hybrids
Prothero: A Rebuttal
Branches of Biology
Dog-fox Hybrids