On the Origins of New Forms of Life
4.9: Forms Capable of Vegetative Reproduction
(Continued from the previous page)
Vegetative reproduction allows new forms of hybrid origin to propagate and persist even when absolutely sterile. Most plants can reproduce vegetatively by sprouting and fragmentation. In animals, equivalent processes are known as splitting and regeneration. Budding occurs in both animals and plants. Vegetative reproduction also occurs in most fungi.
One example of this type of stabilization process has already been given (see above), the vegetatively-reproducing natural hybrids between the ryes Leymus triticoides and L. condensatus. These hybrids, which are treated as a species (L. multiflorus), occur in southern California. Though seed sterile, they have spread vegetatively throughout much of the vast San Joaquin Valley.
Walter (1977) gives another example of a highly sterile grass (Holcus mollis) of probable hybrid origin propagating itself by vegetative reproduction. The widely cultivated edible banana Musa paradisiaca (plantain) is a sterile seedless hybrid from the cross M. acuminata × M. balbisiana (Simmonds 1966, 1976). Although incapable of sexual reproduction, it is propagated vegetatively by offshoots.
Bartley's Clubmoss (Huperzia bartleyi), a natural hybrid, was produced by the cross H. lucidula × H. porophila (Shining Clubmoss × Rock Clubmoss).1 It is sexually sterile but widespread in the eastern United States. It reproduces by means of wind-dispersed shoots.2

|
| Equisetum moorei — A hybrid of Equisetum hyemale and Equisetum ramosissimum Photo: Petr Filippov |
Werth and Wagner (1990: 701) note that among the horsetails (Equisetaceae) there are a number of examples of sterile somatypes treated as species that are of hybrid origin. They are rendered reproductively competent by vegetative reproduction from stem fragments. Werth and Wagner give the example of Ferris' Horsetail (Equisetum ferrissii), a hybrid derived from the cross Common Horsetail × Smooth Horsetail (E. hyemale × E. laevigatum). They say it
The bittercress Caramine insueta is a natural triploid hybrid derived from C. rivularis and C. amara. It arose in the vicinity of Urnerboden, Switzerland, sometime during the twentieth century through the fertilization of a diploid C. rivularis egg cell by haploid C. amara pollen. These hybrids are highly sterile, producing only 2–3 percent good pollen. Yet Grant (1981: 409) says
An agamospermous life cycle, because it does not ordinarily involve meiosis,4 does not produce genetic variability. However, since many organisms having such a life cycle also have a sexual life cycle, the same sources of variation are available to them as to organisms that are strictly sexual. Sexual reproduction may be impaired in such organisms, but still occur at low rates. When vegetative reproduction is combined with low levels of sexual reproduction, whole complexes of new forms can arise.
In the southwestern United States, walkingstick cactus (Cylindropuntia spinosior) and jumping cholla (C. fulgida) produce F₁ hybrids that are highly seed-sterile but have a great capacity for vegetative reproduction via fallen stem joints.5 vegetative reproduction by these hybrids has produced a stable somaset of clonal descendants identical to the F₁ founders. Over evolutionary time, rare sexual reproduction by members of this F₁-identical somaset occasionally produces later-generation hybrids, each of which founds a morphologically distinct clonal somaset propagated by vegetative reproduction (recall that, although F₁ hybrids are generally uniform, their gametes are highly variable, see Section 2). Many of these later-generation hybrids are morphologically distinct (as is usual with variable later-generation hybrids produced by interbreeding between distinct parental somasets). Each distinct somatype among the later-generation hybrids produces via vegetative reproduction a distinct clonal somaset of invariant descendants (Figure 4.4).
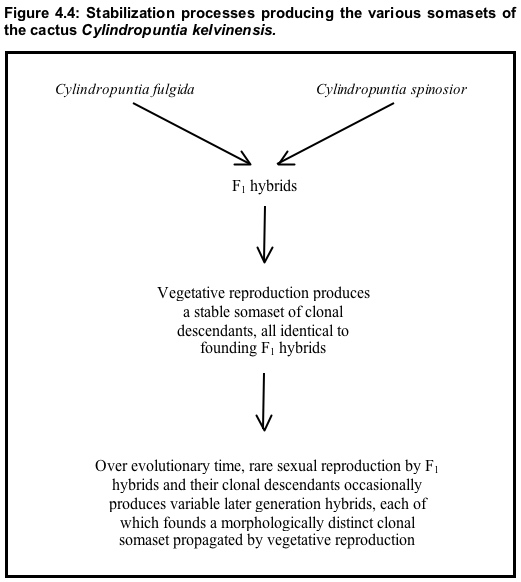
In addition, each of these clones, if sexually fertile in any degree whatsoever, can on rare occasion, found additional types of clones via sexual reproduction (as is the case with the F₁ clones). This process has produced an abundant population of hybrids in the Gila Valley in southwestern Arizona. Grant (1981: 465) notes that one of the clonal somasets in that hybrid population "covers an area 20 miles long by 26 miles wide in the desert." He also says (ibid) that because the hybrid population forms "a distinctive element in the Arizona flora" it is treated as a species, C. kelvinensis.6 Pinkava et al. (1985: 472-473) note that the predominant somatype within the polytypic C. kelvinensis is a triploid and that evidence strongly suggests that C. fulgida provided the unreduced gamete producing this new chromotype. Hence, the primary somaset of C. kelvinensis was produced by a chromosomal mutation. NEXT PAGE >>
Notes (Works Cited):
1. Werth and Wagner (1990: 700).
2. Werth and Wagner (1990: 700).
3. Werth and Wagner cite Hauke (1963) and Wagner and Hammitt (1970).
4. White (1973a: 696); Suomalainen et al. (1987).
5. Grant and Grant (1971a); Kearney and Peebles (1942, 1964).
6. See also: Benson (1950, 1969, 1982); Benson et al. (1940); Kearney and Peebles (1942, 1964); Peebles (1936).
Most shared on Macroevolution.net:
Human Origins: Are we hybrids?
On the Origins of New Forms of Life
Mammalian Hybrids
Cat-rabbit Hybrids: Fact or fiction?
Famous Biologists
Dog-cow Hybrids
Georges Cuvier: A Biography
Prothero: A Rebuttal
Branches of Biology
Dog-fox Hybrids